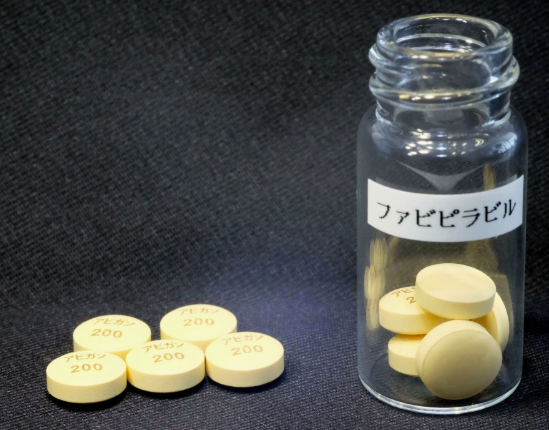
The Japanese government is planning an unprecedentedly short screening period of three weeks once Fujifilm Toyama Chemical Co submits a new drug application.
- That submission is expected in mid-October
- Which would thus lead to approval of Avigan as a coronavirus treatment in November
The news comes via the Japanese media, citing unnamed sources.
---
There will, of course, be concerns about the scrutiny efficacy and safety of the drug with such a fast-track process. Keep in mind also that back in May then PM Abe said approval for Avigan was expected that month.
---
Meanwhile there is a lot of focus on how Trump is faring with his infection. He has posted a video on Twitter saying he is starting to feel good, will be back soon, and what have you. Gosssip is swirling about how believable it is. Interpretations seem to split along political lines, unsurprisingly. A lot of heat, not much light.
I posted back on Friday a potential timeline for developments to watch:



